This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Successful completion of the OPPIK project ” Transfer factor – research and development of a new dosage form of immunotherapeutic medicinal product “
This year, company AUMED a.s. successfully completed a multi-year project of the OPPIK program entitled “Transfer factor – research and development of a new dosage form of immunotherapeutic medicinal product” (project number CZ.01.1.02/0.0/0.0/16_083/0010301).
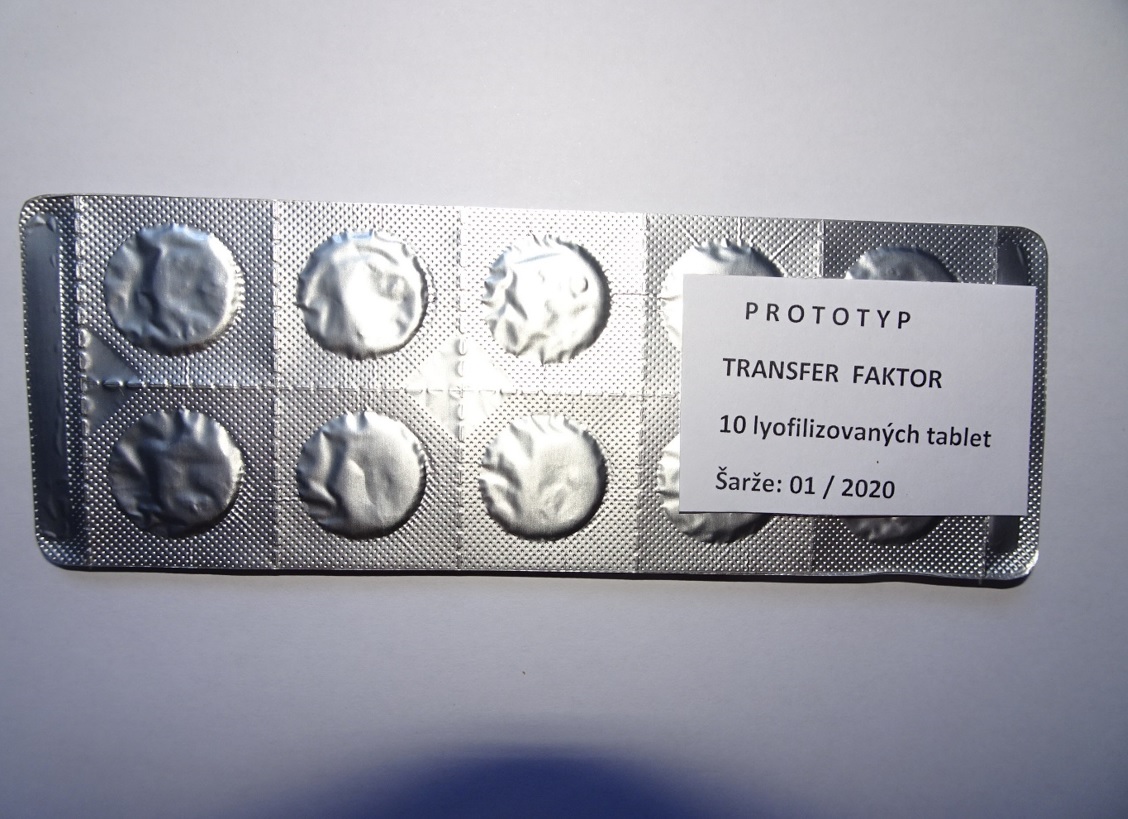
The subject of the project was preparation of a new dosage form of specific immunomodulators based on transfer factors (TF) and methods of testing their immunobiological potential. The research and development of the final product (lyophilized tablets) was completed. The final product was transferred from the laboratory preparation mode to the production – technological arrangement. Experimental samples were made in the form of lyophilized tablets and tested for biological efficacy. The result of the prototype and pilot plant was completed.
The main benefit of the project is an innovatively enhanced medical product of specific immunomodulators based on human transfer factors (TF) administered orally as a lyophilized tablet, which solves both painful administration in the first case and influencing the efficacy of transfer factors by the digestive system when administered orally in liquid form. The composition of the medicinal product is a dialysate of peripheral blood leukocyte homogenate, a physiologically balanced mixture of biologically active substances that occur naturally in the body and perform specific functions in the immune system. Human leukocytes are a natural tool of the immune system. The main mission of this drug is to normalize cellular immunity by transferring specific cellular immunity from the donor to the recipient. New commercially-interesting medication is fully developer and ready for registration!
The medical product is designed for treatment primarily in cases of laboratory-proven cellular immune deficiency, as well as in clinical cases of diseases known to be usually associated with cellular immune deficiency. It will also be indicated in humoral immune deficiencies with laboratory evidence of decreased immunoglobulin levels, and fluctuating findings of impaired cellular immunity, with relapsing infections of various locations.