This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Diagnostics of Covid-19 using RT-LAMP method
AUMED a.s. is involved in the fight against the Covid-19 pandemic. In cooperation with Protean s.r.o. and Institute of Molecular and Translational Medicine, AUMED a.s. launched a modern diagnostics of SARS-CoV-2 virus under the name AUMED test RT-LAMP Assay SARS-CoV-2. It is a diagnostic using the RT – LAMP Assay method. The test uses the so-called LAMP reaction, which is isothermal amplification of nucleic acids. Unlike the polymerase chain reaction (PCR, qPCR), in which the reaction is performed using temperature cycles, isothermal amplification is performed at a constant temperature so there is no require for any expensive equipment. The results are reliably read based on the color change with the naked eye.
WHY LAMP REACTION?
Molecular amplification technique RT-LAMP (Reverse transcription – Loop mediated isothermal amplification) allows the amplification of nucleic acids with high specificity, efficiency and speed under isothermal conditions – i.e. at a constant temperature, achievable in incubators, hybridization ovens or dryers.
Main advantages of AUMED test RT – LAMP Assay SARS-CoV-2:
• No need for expensive laboratory equipment – can be performed in any laboratory, no PCR cycler required.
• Results are available within one hour – the possibility of reading with the naked eye.
• Hundreds of samples can be analyzed at once.
• Reduced costs compared to conventional PCR tests with the same sensitivity and reliability.
• The entire test can be performed in a small or mobile laboratory, possibly connected directly to the sampling site.
• Ideal solution for airport terminals, large companies, border crossings, homes for the elderly…
• The use of natural amplification enzymes guarantees high reliability of the test results, so that even if the incubation time is accidentally exceeded, false positive results do not occur. Competitive LAMP assays that use genetically engineered enzymes with higher processivity suffer from this problem. These tests are slightly faster, but there is a risk of false positive results.
DIAGNOSTIC KIT
The kit is fully validated and registered by the State Institute for Drug Control (SÚKL) under registration number 00912050 (issued by SIDC-CZ). Instructions and a valid declaration of conformity can be downloaded from the appendix of this registration. It is a completely Czech product without dependence on foreign suppliers.
The kit is intended for diagnosis of the presence of SARS-CoV-2 virus in isolated viral RNA samples – for 45 samples. The diagnostic kit involves the detection of SARS-CoV2 virus and the detection of an internal control (IC) to check the correct collection and processing of the sample.
The functional evaluation was successfully performed by the Institute of Molecular and Translational Medicine in Olomouc. Comparison of the kit with the reference RT-PCR assay determined the sensitivity of the assay to be 93% and specificity to 99%. The kit passed all tested parameters and was recommended for use.
Test sensitivity:
• Equivalent sensitivity with qPCR up to Ct 36-38
• Corresponds to 10 copies of virus per ul sample
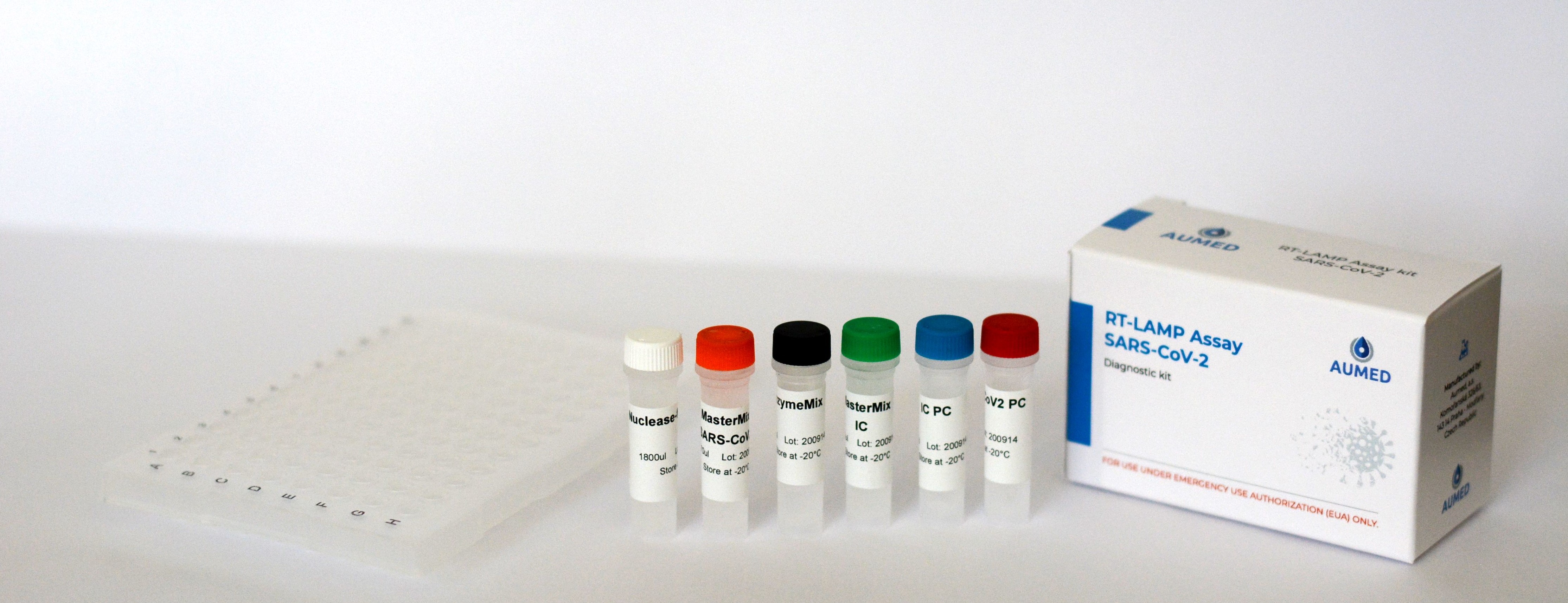
Kit contents:
• A bag containing a 96-hole PCR plate and a plate of colored caps in a light-tight package. Should be stored at room temperature, caps in the original light-tight package or in the dark.
• Reagent box for 45 tests
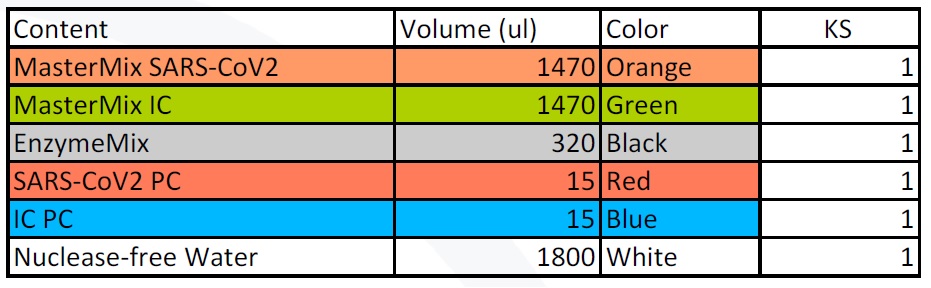
IC = internal control; PC = positive control
Material required – not supplied with the kit:
• Automatic and multichannel pipettes
• Appropriate reservoir for the reaction mixture
• Pipette tips with RNAse and DNAse free aerosol filters
• Plate incubation device at 63 °C
NEW SAMPLING SYSTEM
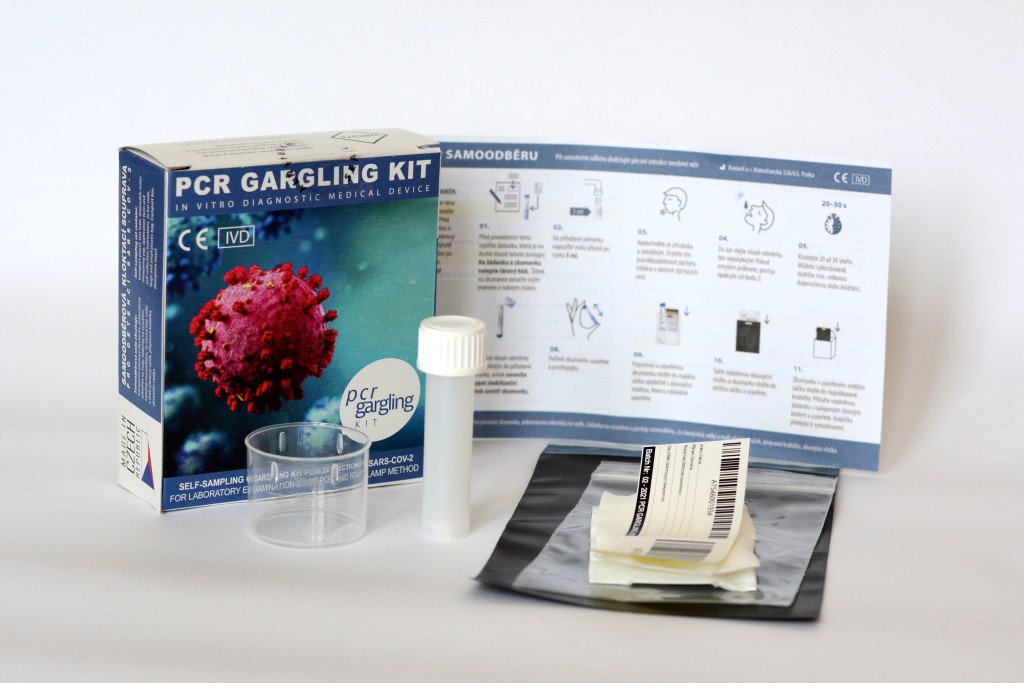
AUMED, a.s. launches its own self-sampling set based on the principle of gargling of the oral cavity and pharynx. The new sampling set was named PCR GARGLING KIT. The self-sampling device is intended for collecting samples, which are then tested in a professional laboratory for the presence of viruses infecting the oral cavity and upper respiratory tract. The kit was primarily developed for the detection of SARS-CoV-2 virus, which causes covid-19. The evaluation is performed in the laboratory by detection based on nucleic acid amplification such as RT-PCR and RT-LAMP methods. The kit is fully validated and meets CE IVD certification. The kit is registered by the State Institute for Drug Control (SÚKL) under registration number 00959967 (issued by SIDC-CZ).
The new collection is therefore much more pleasant and completely painless. Instead of pushing the sticks into the nose, it is enough to gargle a sip of ordinary water for 20-30 seconds and then empty this content from the mouth into the enclosed collection container containing the transport powder. The user then closes the tube and packs it according to the instructions. The user will perform this procedure either at the sampling point or at home, from where he will then deliver the sample to the sampling point, to the laboratory or, for example, to his doctor.
The combination of the new PCR GARGLING KIT sampling set and the AUMED test RT-LAMP Assay SARS-CoV-2 test kit creates completely new possibilities for the detection of new coronavirus in the population. Painless and fast collection without queues is followed by fast and reliable diagnostics!
AUMED a.s. ensures the production of the sampling set. If you are interested, contact us by email info@aumed.cz and we will contact you immediately with a price offer depending on the number of required sets.
DO YOU WANT TO TRY OUR TEST? WE OFFER A COMPREHENSIVE SOLUTION FOR PREVENTIVE TESTING
The diagnostic kit can be ordered by email at info@aumed.cz. We will reply to your email immediately and you will find out everything important about prices and delivery dates.
AUMED a.s. offers for companies a comprehensive solution for preventive testing of the SARS-CoV-2 virus (COVID-19). We provide sampling and subsequent testing of samples. We perform sampling with the PCR GARGLING KIT self-sampling set, the principle of which is based on gargling of the oral cavity and pharynx. The collection is therefore much more pleasant and completely painless. Instead of pushing the sticks into the nose, it is enough to gargle a sip of ordinary water for 20-30 seconds for half a minute and then empty this contents from the mouth into the enclosed collection tube containing the transport powder. The evaluation is then performed by our newly developed test kit using the RT-LAMP method. The RT-LAMP test is functionally comparable to the RT-PCR test. For long-term and effective protection of the company, therefore, a significantly longer interval of repeated testing will suffice than in the case of antigen tests, where retestation occurs every 5 days! This makes our testing more cost-effective than inaccurate antigen testing. We send the results within 6-8 hours after receiving the samples. If you are interested, please contact us at info@aumed.cz.
For summary information and instructions for use, visit the website www.aumedtest.cz dedicated to this diagnostic kit
The production of the AUMED test RT-LAMP Assay SARS-CoV-2 diagnostic kit was made possible also thanks to the support of the Operational Program for Entrepreneurship and Innovation for Competitiveness. Specifically, AUMED a.s. received support for the project PRODUCTION OF DIAGNOSTIC KIT FOR COVID-19 ON THE LAMP PRINCIPLE within the TECHNOLOGY SUPPORT PROGRAM – CALL XII. COVID 19 (OP: Entrepreneurship and Innovation for Competitiveness 2014-2020, call number: XII, program call: Technology). Part of the project was the purchase of technological equipment needed for the production of this diagnostic kit.



